Clinical data management
Clinical data management
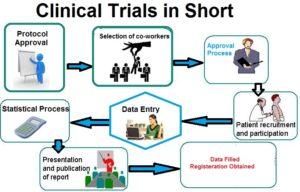
CDM is a critical phase in clinical research, which leads to generation of high-quality , reliable, and statistically sound data from clinical Studies.
- Database Design (eCRF entry screen) & its specification on CDMS tool as per CDASH Standards.
- Data validation plan (Edit check programming) & programmed the edit check as expected query check.
- Perform test data for User Acceptance Testing (UAT).
- Data Management Plan development .
- Data entry guideline, Data specification(DS), database lock checklist, Data Export etc
- Perform Medical Coding for AE and Concomitant Medication in CDM Tool.